Ahead of new clinical readouts at ASCO 2025, we utilized our AI-driven probability of technical and regulatory success (PTRS) assessments to identify oncology programs with strong potential, informed by both model insights and internal scientific expertise, to illustrate the predictive power of PTRS as an early indicator of success.
We identified a focused set of biopharma programs expected to yield noteworthy updates during the 2025 ASCO conference. These selections reflected a data-driven view of clinical momentum and innovation potential, signaling where the industry’s scientific and investment attention might be most effectively directed.
Now that ASCO 2025 has come to a close, we revisited our watchlist-worthy companies before ASCO: Allogene Therapeutics, BioAtla, Actuate Therapeutics, Zai Lab Limited, and Perspective Therapeutics.
So, how did we do? In short, our pre-ASCO predictions came to fruition as all five delivered meaningful, positive clinical outcomes and stood out among peer programs, validating the precision of our predictive methodology.
Our Data, Methodologies and Expertise Were Validated
The subsequent validation of these predictions through the publication of positive clinical outcomes at ASCO reinforces the commercial and operational value of our AI-driven approach. For R&D leaders, investors, and business development teams, this outcome highlights the growing utility of predictive modeling not just as a scientific tool, but as a strategic asset—enabling better portfolio prioritization, smarter partnering and more informed pipeline bets in an increasingly competitive oncology landscape. We took a similar approach both before and after ASH 2024, which yielded positive outcomes, again validating our data, methodology, and expertise.
As with previous initiatives, we employed our established methodology—anchored in the probability of technical and regulatory success (PTRS) model—to evaluate clinical-stage oncology programs ahead of ASCO 2025. This year, the effort expanded beyond hematologic malignancies to encompass the full oncology landscape, reflecting the broader diversity of innovation presented at the meeting.
To build our ASCO 2025 watchlist, we began with the complete universe of companies currently advancing active oncology programs. From this broad dataset, we applied a series of filters, excluding large pharmaceutical sponsors to focus on emerging innovators, prioritizing those likely to present at ASCO, and finally ranking candidates using our proprietary PTRS assessment. This process yielded a refined list of 20 high-potential biotechs.
From there, we identified five standout companies by selecting programs with the highest PTRS within their respective peer groups, supplemented by domain-specific expert insights to validate scientific and clinical relevance.
The output was five watchlist-worthy companies: Allogene Therapeutics, BioAtla, Actuate Therapeutics, Zai Lab Limited and Perspective Therapeutics. In our pre-ASCO watchlist, you’ll get a deeper look into our methodology and rationale for each of these selected companies.
Validated Foresight: ASCO 2025 Outcomes Aligned With Predictive Insights
Following the close of ASCO 2025, we revisited the five companies spotlighted in our pre-conference analysis. The results were unequivocal: all five delivered meaningful, positive clinical outcomes, validating the precision of our predictive methodology.
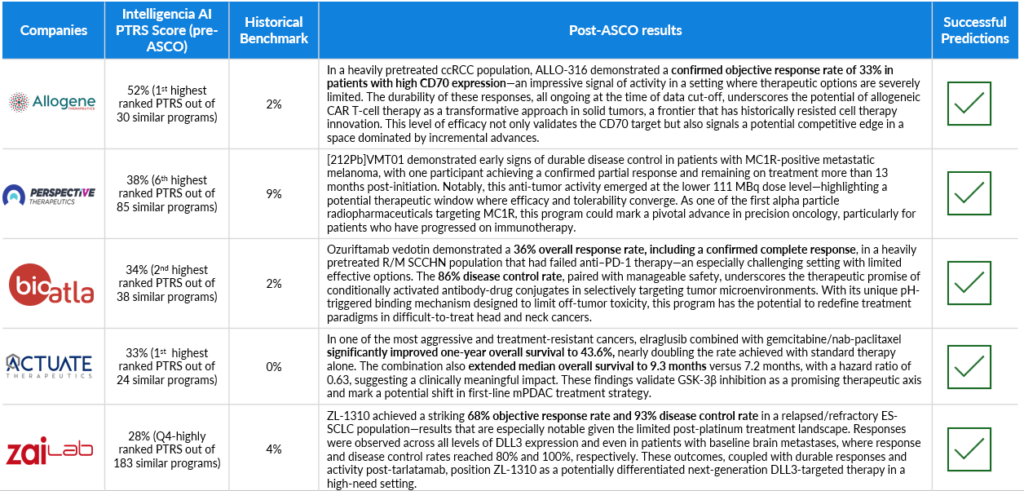
Company: Allogene
Intelligencia AI PTRS (pre-ASCO): 52% (1st highest ranked PTRS out of 30 similar programs)
Historical Benchmark: 2%
Post-ASCO Results: In a heavily pretreated ccRCC population, ALLO-316 demonstrated a confirmed objective response rate of 33% in patients with high CD70 expression—an impressive signal of activity in a setting where therapeutic options are severely limited. The durability of these responses, all ongoing at the time of data cut-off, underscores the potential of allogeneic CAR T-cell therapy as a transformative approach in solid tumors, a frontier that has historically resisted cell therapy innovation. This level of efficacy not only validates the CD70 target but also signals a potential competitive edge in a space dominated by incremental advances.
Successful Prediction: Yes
Company: Perspective Therapeutics
Intelligencia AI PTRS (pre-ASCO): 38% (6th highest ranked PTRS out of 85 similar programs)
Historical Benchmark: 9%
Post-ASCO Results: [212Pb]VMT01 demonstrated early signs of durable disease control in patients with MC1R-positive metastatic melanoma, with one participant achieving a confirmed partial response and remaining on treatment more than 13 months post-initiation. Notably, this anti-tumor activity emerged at the lower 111 MBq dose level—highlighting a potential therapeutic window where efficacy and tolerability converge. As one of the first alpha particle radiopharmaceuticals targeting MC1R, this program could mark a pivotal advance in precision oncology, particularly for patients who have progressed on immunotherapy.
Successful Prediction: Yes
Company: BioAtla
Intelligencia AI PTRS (pre-ASCO): 34% (2nd highest ranked PTRS out of 38 similar programs)
Historical Benchmark: 2%
Post-ASCO Results: Ozuriftamab vedotin demonstrated a 36% overall response rate, including a confirmed complete response, in a heavily pretreated R/M SCCHN population that had failed anti–PD-1 therapy—an especially challenging setting with limited effective options. The 86% disease control rate, paired with manageable safety, underscores the therapeutic promise of conditionally activated antibody-drug conjugates in selectively targeting tumor microenvironments. With its unique pH-triggered binding mechanism designed to limit off-tumor toxicity, this program has the potential to redefine treatment paradigms in difficult-to-treat head and neck cancers.
Successful Prediction: Yes
Company: Actuate Therapeutics
Intelligencia AI PTRS (pre-ASCO): 33% (highly ranked PTRS out of 24 similar programs)
Historical Benchmark: 0%
Post-ASCO Results: In one of the most aggressive and treatment-resistant cancers, elraglusib combined with gemcitabine/nab-paclitaxel significantly improved one-year overall survival to 43.6%, nearly doubling the rate achieved with standard therapy alone. The combination also extended median overall survival to 9.3 months versus 7.2 months, with a hazard ratio of 0.63, suggesting a clinically meaningful impact. These findings validate GSK-3β inhibition as a promising therapeutic axis and mark a potential shift in first-line mPDAC treatment strategy.
Successful Prediction: Yes
Company: Zai Lab
Intelligencia AI PTRS (pre-ASCO): 28% (top-ranked PTRS out of 183 similar programs)
Historical Benchmark: 4%
Post-ASCO Results: ZL-1310 achieved a striking 68% objective response rate and 93% disease control rate in a relapsed/refractory ES-SCLC population—results that are especially notable given the limited post-platinum treatment landscape. Responses were observed across all levels of DLL3 expression and even in patients with baseline brain metastases, where response and disease control rates reached 80% and 100%, respectively. These outcomes, coupled with durable responses and activity post-tarlatamab, position ZL-1310 as a potentially differentiated next-generation DLL3-targeted therapy in a high-need setting.
Successful Prediction: Yes
Each selected program ranked among the highest-performing assets within its peer group for the same indication and development stage. Differentiated mechanisms, strong scientific rationale, and clear potential for clinical impact characterized these candidates. It’s important to highlight that our model identified them as top-tier programs well in advance of any public data disclosures.
We’ve had a strong track record in such assessments (ASH 2024), which reinforces the credibility and strategic value of the combination of data, AI and expert insights —not only as a forecasting tool, but as a trusted decision-making asset that can enhance confidence in early-stage opportunity assessment and portfolio planning.
Why It Matters: Unlock Strategic Advantage With Predictive Precision
In today’s dynamic R&D environment, timely and trusted insights are crucial for making informed, high-stakes decisions throughout the drug development lifecycle. Our accurate ASCO predictions serve as a compelling demonstration of how data-driven foresight can de-risk decision-making and drive measurable value.
Accelerate Portfolio Prioritization
Rapidly surface the most promising programs within your pipeline, enabling focused allocation of resources toward assets with the highest likelihood of success—maximizing both efficiency and return on investment.
Inform Intelligent Capital Deployment
Rely on AI-powered, evidence-based forecasts to reduce uncertainty and guide smarter investment decisions, whether in R&D, licensing, or strategic partnerships.
Validate Predictive Performance
Our PTRS platform consistently identifies breakout programs ahead of public disclosures, offering stakeholders a trusted source of actionable insight rooted in analytical rigor.
Gain Early-Mover Advantage
Have the critical edge with preemptive visibility into high-impact opportunities—positioning your organization ahead in a competitive market increasingly defined by speed, precision, and innovation.
