The European Society for Medical Oncology (ESMO) annual congress has become a premier stage for unveiling oncology data that can transform both clinical practice and commercial strategy. It is a venue where early scientific insights frequently set the tone for treatment innovation and industry investment.
At Intelligencia AI, our core mission is to advance drug development through data-driven analysis and expert insight. With our unique vantage point of combining proprietary data assets, AI-powered analytics, and domain expertise, we pinpointed programs that appear especially noteworthy and promising. Sharing these insights is a natural extension of our work, providing stakeholders with a clearer view of where innovation may have a meaningful impact.
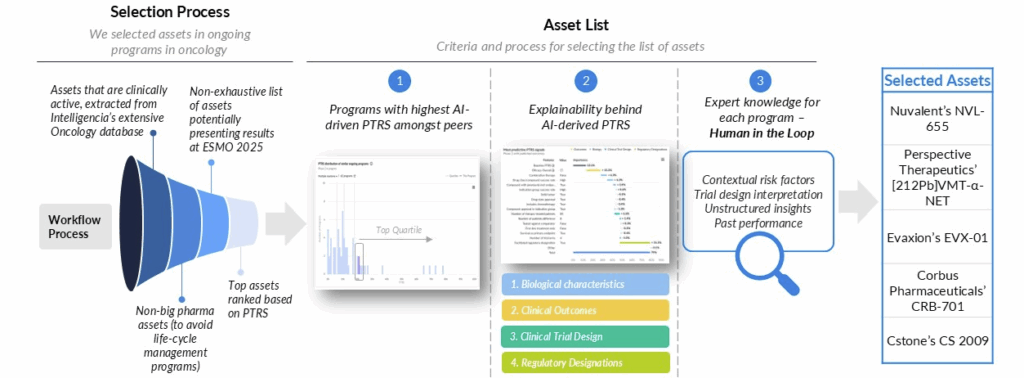
Methodology
Our watchlist methodology is grounded in Intelligencia AI’s Probability of Technical and Regulatory Success (PTRS) framework, which combines quantitative modeling with expert interpretation to identify the most promising oncology programs.
Step 1: Asset Identification
We begin with the full universe of actively developing oncology assets from our oncology database. Programs sponsored by large pharmaceutical companies (>$10B market cap) are excluded to focus on emerging innovators rather than life-cycle management plays.
Step 2: PTRS-Based Ranking
Within this refined universe, we apply our AI-driven PTRS model. The model incorporates biological characteristics, clinical outcomes, trial design features, and regulatory designations. It’s further enriched with unstructured insights and contextual risk factors. This ensures a balanced view of both opportunity and risk.
Step 3: Expert Validation (“Human in the Loop”)
Our on-staff scientific and clinical experts review the model-driven outputs to contextualize signals, interpret trial design nuances, and incorporate domain knowledge that may not be fully captured in quantitative data.
Step 4: Watchlist Selection
From this integrated process, we selected a list of assets most likely to generate clinically meaningful and strategically significant readouts. Programs highlighted typically score among the highest in PTRS relative to peers, while also standing out for scientific novelty, differentiation, or breadth of potential application.
Workflow Overview
- Identify clinically active assets from Intelligencia AI’s oncology database.
- Apply PTRS modeling to rank programs within peer groups.
- Incorporate expert review to validate clinical and scientific relevance.
- Highlight assets with the greatest potential impact at upcoming conferences.
Consistent Validation of Methodology
This methodology has been stress-tested in prior conferences, including ASCO 2025, where our watchlists correctly anticipated assets that went on to deliver positive, differentiated outcomes.
At ASCO 2025, all five companies identified in our pre-conference analysis—Allogene Therapeutics, BioAtla, Actuate Therapeutics, Zai Lab, and Perspective Therapeutics—subsequently presented meaningful clinical advances. This validated the predictive power of our PTRS methodology as both a scientific and strategic tool.
Our proprietary company score leverages our AI-driven PTRS assessments.
1. NVL-655, a brain-penetrant ALK inhibitor, offers a differentiated approach for patients with ALK+ solid tumors by overcoming resistance and reducing off-target toxicity (Nuvalent)
Indication: Solid cancer
Trial: Phase 1/2, NCT05384626
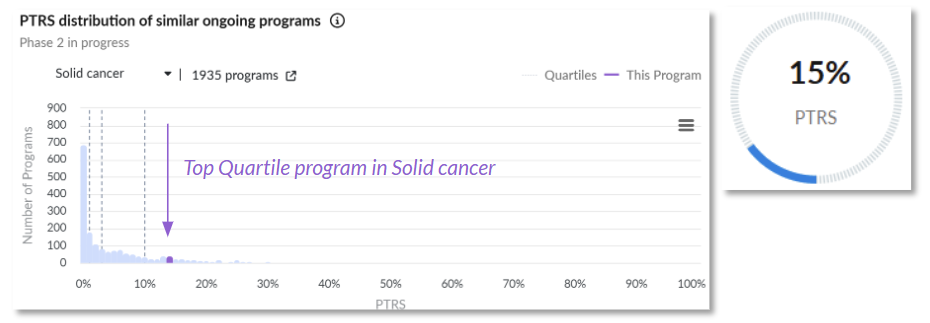
Probability of technical and regulatory success (PTRS) relative to the competitive landscape.
Drug Characteristics:
- Selective ALK Inhibition: NVL-655 is a next-generation ALK tyrosine kinase inhibitor (TKI) designed to maintain potency against common resistance mutations that limit the durability of first-generation agents.
- CNS Penetration: Engineered to achieve brain exposure, NVL-655 addresses a critical unmet need in ALK-positive NSCLC, where CNS metastases are common and poorly controlled by current therapies.
- Favorable Selectivity Profile: By minimizing off-target TRK inhibition, NVL-655 aims to reduce neurological adverse events commonly associated with earlier ALK inhibitors, potentially supporting improved tolerability and adherence.
Positive Clinical Trial Characteristics: While no efficacy outcomes have been published for the trial’s solid cancer population, NVL-655 demonstrated objective responses of ~38 % in heavily pretreated ALK-positive NSCLC patients (including those who had progressed on multiple prior ALK TKIs, including lorlatinib).
This is quite promising for further development, and given the above drug characteristics, there is reason to believe the broader solid tumor population could also derive meaningful benefit.
Competitive Positioning: Within the ALK treatment landscape, NVL-655 distinguishes itself by its dual focus on resistance and brain penetration. Competitors have struggled to achieve both, leaving an opportunity for NVL-655 to redefine standards.
Why It Matters: With novel biology, strong PTRS signals, and a strategically designed trial, NVL-655 is a prime candidate to attract attention at ESMO 2025.
2. [²¹²Pb]VMT-α-NET delivers targeted alpha therapy (TAT) to somatostatin receptor–positive NETs, combining precision radiopharmaceutical design with theranostic capability (Perspective Therapeutics)
Indication: Neuroendocrine carcinoma
Trial: Phase 1/2, NCT05636618
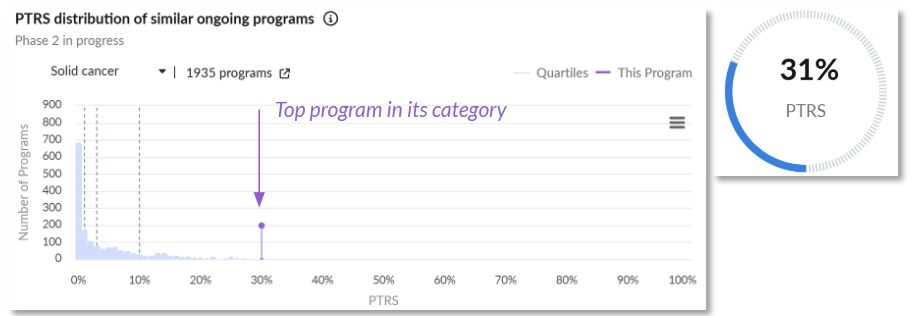
Probability of technical and regulatory success (PTRS) relative to the competitive landscape.
Drug Characteristics:
- Targeted Alpha Therapy: [²¹²Pb]VMT-α-NET is a radiopharmaceutical designed to deliver alpha-emitting isotopes directly to NET cells expressing somatostatin receptors, enabling highly localized tumor cell killing while sparing surrounding tissue.
- Theranostic Approach: The ligand can be paired with gamma-emitting isotopes for imaging, providing a way to select patients most likely to benefit and monitor uptake in real time.
- High-Energy, Short-Path Killing: Alpha particles from ²¹²Pb produce dense DNA damage within a limited range, potentially overcoming resistance mechanisms that blunt the effects of beta-emitting therapies such as lutetium-based agents.
Clinical Trial Outcomes: The program is rewarded for the positive interim efficacy results that have already been announced, as well as the number of successfully enrolled patients. Among patients treated at the escalation stage, a 33% ORR was achieved.
Regulatory Designations: The program received a Fast-Track designation in October 2022, which is a major positive predictor for a program this early in development.
Competitive Positioning: Among TAT programs in development, [²¹²Pb]VMT-α-NET is one of the most advanced and targeted. Its combination of potency, precision, and theranostic capability positions it as a frontrunner in the neuroendocrine tumor (NET) space.
Why It Matters: With radiopharmaceuticals gaining momentum, [²¹²Pb]VMT-α-NET exemplifies how alpha therapy may advance the field beyond current beta-emitting approaches.
3. EVX-01 is a personalized neoantigen vaccine designed to expand immune recognition in melanoma, offering synergy with checkpoint inhibition (Evaxion)
Indication: Melanoma
Trial: Phase 2, NCT05309421
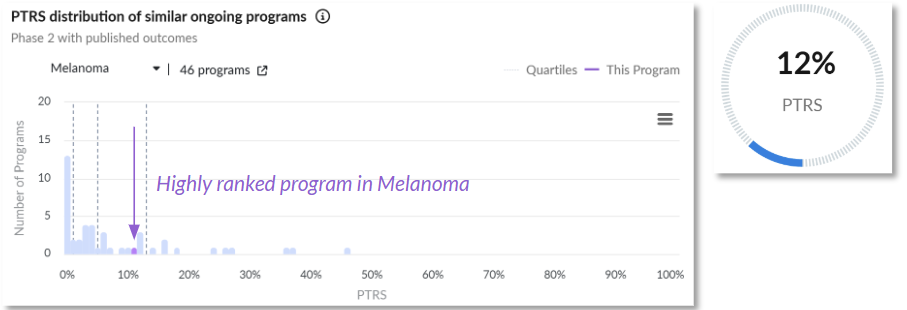
Probability of technical and regulatory success (PTRS) relative to the competitive landscape.
Drug Characteristics:
- Personalized Cancer Vaccine: EVX-01 is a neoantigen-based immunotherapy, developed using AI to identify patient-specific tumor mutations most likely to trigger a robust T-cell response.
- Synergy with Checkpoint Blockade: Designed to complement PD-1 inhibitors, EVX-01 has the potential to enhance both breadth and depth of immune responses in advanced melanoma.
- Adaptive Platform: By tailoring vaccine composition to each patient’s mutational landscape, EVX-01 exemplifies a precision approach to immuno-oncology with potential scalability beyond melanoma.
Clinical Trial Outcomes: EVX-01 demonstrated a 69% overall response rate (ORR) in 11 of 16 patients at the one-year interim point; 15 of 16 patients experienced measurable reductions in target lesions.
Competitive Positioning: While cancer vaccines have historically faced challenges, EVX-01 leverages technological innovation to overcome past barriers. Its personalization and synergy with immunotherapy differentiate it in a crowded immuno-oncology pipeline.
Why It Matters: EVX-01 has the potential to validate personalized cancer vaccines as a viable adjunct to checkpoint therapy, a milestone many in the field have long anticipated.
4. CRB-701 leverages Nectin-4 targeting with a refined ADC design, aiming to deliver potent, selective therapy across diverse solid tumors (Corbus Pharmaceuticals)
Indication: Solid cancer
Trial: Phase 1/2, NCT06265727
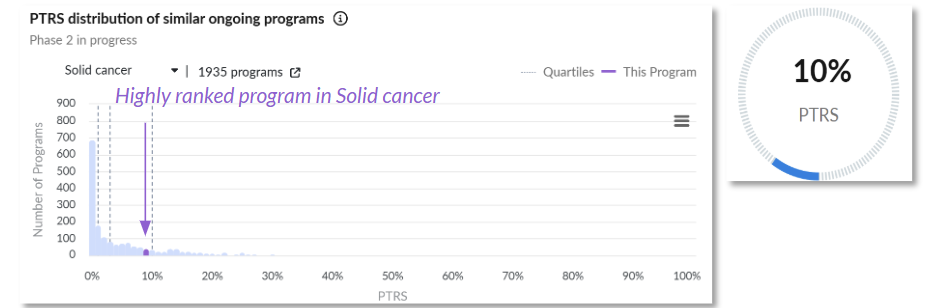
Probability of technical and regulatory success (PTRS) relative to the competitive landscape.
Drug Characteristics:
- Nectin-4 Targeting ADC: CRB-701 is an antibody-drug conjugate directed against Nectin-4, a cell adhesion molecule highly expressed in multiple solid tumors, including urothelial carcinoma and breast cancer.
- Proven Payload Design: The ADC employs a monomethyl auristatin E (MMAE) payload linked via a cleavable linker, enabling targeted delivery of a potent cytotoxic agent with a well-understood mechanism of action.
- Broad Applicability: Given Nectin-4’s expression across tumor types, CRB-701 has the potential for therapeutic reach beyond its lead indication, opening avenues for expansion across diverse solid cancers.
Clinical Trial Outcomes: The program is rewarded for the positive interim efficacy results that have already been announced, as well as the number of enrolled patients. This program achieved an ORR of 26.7%, including mostly partial responses.
Competitive Positioning: Within the ADC landscape, CRB-701 benefits from lessons learned with earlier agents while advancing the field with improved design. Its differentiation lies in balancing potency with selectivity.
Why It Matters: Given the surge in interest in ADCs, CRB-701 is poised to demonstrate how platform refinements can lead to significant clinical differentiation.
5. With its trispecific design, CS2009 brings together PD-1, CTLA-4, and VEGFA blockade to enhance immunotherapy in solid tumors (CStone Pharmaceuticals)
Indication: Solid cancer
Trial: Phase 1/2, NCT06741644
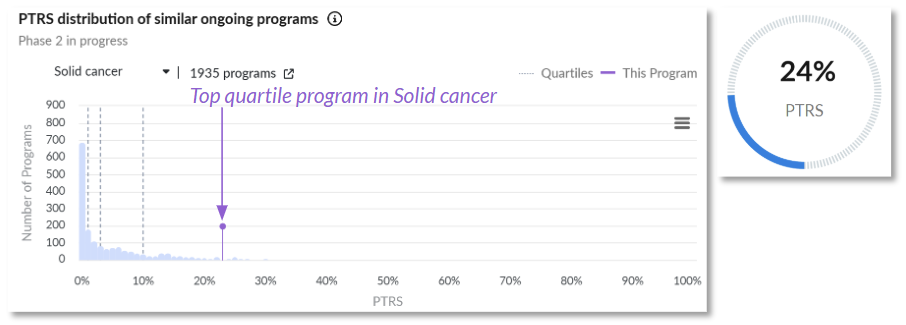
Probability of technical and regulatory success (PTRS) relative to the competitive landscape.
Drug Characteristics:
- Trispecific Antibody Targeting PD-1, CTLA-4, and VEGFA: CS2009 is engineered to simultaneously block PD-1 and CTLA-4 while neutralizing VEGFA, aiming to combine immune checkpoint inhibition with anti-angiogenic activity.
- Avidity-Driven Tumor Selectivity: Its design includes balanced and monovalent arms for PD-1 and CTLA-4, plus a bivalent VEGFA arm, which allows preferential targeting of tumor-infiltrating T cells expressing both PD-1 and CTLA-4. This may reduce systemic exposure and potential toxicity.
- Synergy with VEGFA in the Tumor Microenvironment: Preclinical data show that VEGFA crosslinking enhances the immune checkpoint blockade effect of the PD-1/CTLA-4 arms, potentially boosting activity in VEGFA-rich, immunosuppressive tumor environments.
Positive Clinical Trial Characteristics: The program is rewarded for the number of successfully enrolled patients. The asset’s target selection also combines some of the most successful targets in Oncology. While this is not a guarantee for efficacy, said selection holds significant promise, accompanied by strong pre-clinical signals.
Competitive Positioning: Few programs combine checkpoint inhibition with VEGF blockade in a single molecule. CS2009’s design simplifies combination therapy into a unified product, potentially improving both efficacy and patient convenience.
Why It Matters at ESMO 2025: If successful, CS2009 could represent a new class of trispecific immunotherapies, thereby reshaping the use of checkpoint inhibitors in solid tumors.
Conclusion
The five programs highlighted here demonstrate the diversity and dynamism of innovation expected at ESMO 2025. They span modalities ranging from targeted small molecules to radiopharmaceuticals, vaccines, ADCs, and multispecific antibodies. Collectively, they illustrate the evolving strategies by which oncology drug development is seeking to overcome resistance, enhance precision, and broaden therapeutic impact.
While the eventual clinical outcomes remain uncertain, each program reflects a strong intersection of scientific novelty, clinical design sophistication, and strategic significance. For stakeholders across oncology—clinicians, researchers, and investors alike—these assets deserve close attention as data emerge at ESMO 2025.
For these and other insights, let’s talk.



