A Field Defined by Need and Repeated Failures
Alzheimer’s disease (AD) remains one of the most urgent and under-addressed therapeutic challenges. Despite rising prevalence and decades of research, true clinical breakthroughs have been few and far between. Repeated late-stage failures, minimal differentiation, and a persistent underperformance relative to other therapeutic areas mark the history of Phase 3 development in AD.
While recent approvals of amyloid-targeting monoclonal antibodies represent genuine scientific progress, they also create a higher bar: new agents must now offer either superior efficacy or differentiated safety to justify their place. That bar has proven difficult to clear.
A Pipeline With Momentum — But Not at the Top
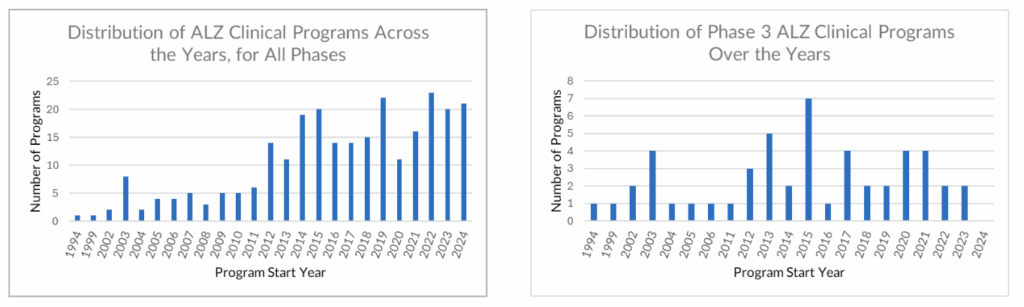
Our analysis tracks 19 active Phase 3 programs in AD, covering 16 unique drugs. At first glance, this reflects continued sponsor commitment. But a closer look reveals a more constrained reality:
- Four of the programs target amyloid-beta, already a validated mechanism with three recent approvals. Differentiation will require clear superiority.
- Three programs revisit the long-explored cholinesterase pathway, which suggests limited innovation.
- The majority focus on non-validated targets, where failure risk is structurally higher.
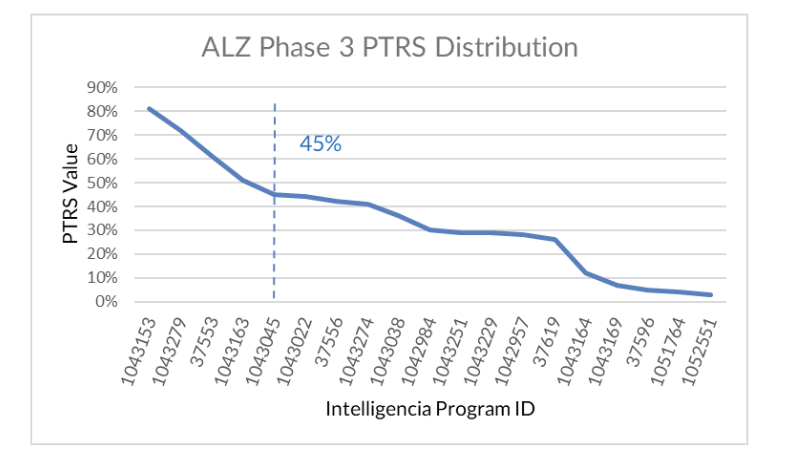
To further assess these programs, we applied our Probability of Technical and Regulatory Success (PTRS ) methodology, a model built on historical outcome data, trial design patterns, and regulatory precedent across therapeutic areas. Even among the five programs that score above average in PTRS, two benefit from prior approvals in other populations, and two rely on “novelty” due to undefined mechanisms. Only one program sits clearly above the average without such caveats.
Risk, Innovation, and a Shrinking Window
Late-stage development in Alzheimer’s faces barriers beyond traditional R&D:
- Disease progression is slow and heterogeneous, making outcomes difficult to measure consistently.
- Trials are long, expensive, and require large patient cohorts with high screen failure rates.
- Biomarkers, while improving, still face questions about their translation into clinical benefit.
This has created an environment where success remains elusive. Despite recent advances, the overall historical rate of approval in Alzheimer’s disease remains just ~4%. Even for programs that reach Phase 3, the success rate only improves to ~14%, still significantly below the Phase 3 average across other therapeutic areas.
To assess how today’s programs stack up within that risk landscape, we applied our data-driven PTRS model by drawing on historical benchmarks across similar programs, trial structures, and mechanisms of action.
We Support Decision-Makers Navigating High-Risk Development
Our SaaS suite of solutions enables deeper signal detection and strategic benchmarking in areas like Alzheimer’s, where few successes exist and historical data is often fragmented.
For clinical development teams, we provide trial design context and outcome comparators that clarify whether a Phase 3 structure is truly differentiated or quietly repeating past patterns.
For portfolio strategists, we offer historical performance benchmarks by mechanism, indication, and trial phase, enabling better timing and investment decisions when the risk curve is steepest.
For business development leaders, we de-risk opportunity assessments by applying predictive analytics to historical outcomes, helping distinguish between unvalidated innovation and just another high-risk bet.
Clarity around what works — and what never did — is the only real advantage in a space where progress is rare.


