In oncology, deciding whether to develop a therapy as a monotherapy or combination is one of the most impactful, strategic choices a company can make. It affects trial design, regulatory complexity, investment requirements and ultimately market access.
Choosing the Path: Key Drivers Behind Treatment Strategy
Regulatory and Clinical Risk: Monotherapies typically face a lower burden of proof and offer clearer efficacy attribution. Combinations must show added value and contribution of components—raising both complexity and risk.
Cost, Speed, and Operational Load: Monotherapy trials often run leaner and faster, especially in early phases. Combinations require larger cohorts, more monitoring, and careful dose management.
Differentiation and Competitive Strategy: In crowded markets, combinations can unlock deeper responses or tackle resistance. Monotherapies may dominate in biomarker-defined or niche settings where a clean signal matters most.
What the Numbers Reveal: Performance by Treatment Type
Historical benchmarks from Intelligencia AI show clear patterns across oncology development programs:
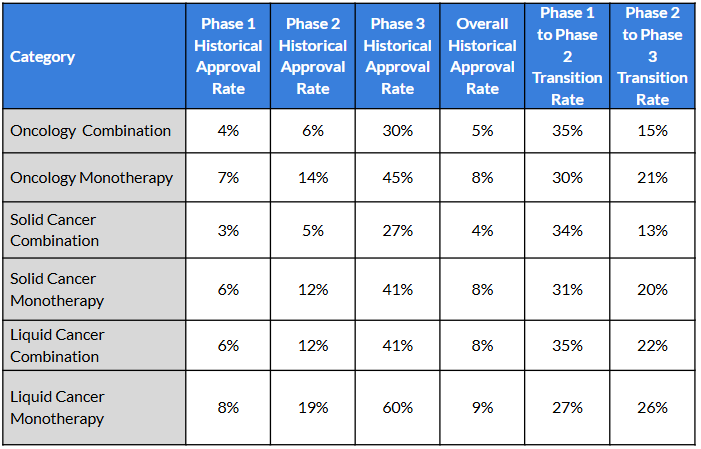
Three Key Insights
1. Monotherapies outperform in late-phase success and overall approval rates, reinforcing their value as capital-efficient paths with strong upside.
2. Combinations transition better from Phase 1, often due to high scientific enthusiasm or synergistic preclinical data, but this early momentum doesn’t always translate to approval.
3. The sharp Phase 3 drop-off for combinations points to common challenges: toxicity stacking, regulatory demands for contribution-of-components and trial complexity.
Why Monotherapies Win More Often
- Focused Risk, Faster Answers: Monotherapies allow early validation or termination, conserving capital and reducing uncertainty.
- Clean Efficacy Signals: Regulatory reviewers respond better to single-agent clarity, especially in diseases with high variability or established SOC.
- Simplified Execution: Fewer variables mean fewer delays, fewer surprises, and faster decision-making across the board.
In short, combinations may bring ambition, but monotherapies often deliver results.
Here’s Where Intelligencia AI Drives Smarter, Strategic Decisions
We empower executives, clinical strategists and portfolio managers with the data and tools to confidently shape treatment design decisions from the outset:
Model trade-offs between monotherapy and combination strategies
Portfolio leaders can use our historical benchmarks and predictive algorithms to compare success probabilities, and risk exposure—helping select the most viable path before major capital is committed.
Inform early-stage go/no-go decisions with phase-specific insight
Our transition data allows decision-makers to evaluate where the risk lies—whether in moving from Phase 1 to 2 or surviving the critical Phase 3 hurdle—based on treatment type, indication and modality.
Build stronger business cases for internal prioritization or external partnerships
Teams can leverage these benchmarks to support data-driven rationales when pitching programs, defending strategy to stakeholders or negotiating co-development agreements.
The right call between monotherapy and combination isn’t guesswork; we ensure it’s grounded in evidence, not assumptions.



