From Breakthrough to Plateau: CAR-T’s First Chapter
Chimeric Antigen Receptor T-cell (CAR-T) therapy remains one of the most transformative innovations in oncology. Known for its precision targeting and durability of response, CAR-T has redefined treatment possibilities—particularly in hematologic malignancies, where multiple therapies have secured approval and demonstrated life-extending benefit in relapsed or refractory patients.
Historically, CAR-T development has focused almost exclusively on liquid cancers, where access to target cells is more straightforward and tumor microenvironments are less suppressive. The field has grown rapidly, but in recent years, clinical activity in liquid tumors has plateaued, and new programs appear to be leveling off.
Solid Tumors Step Into the Spotlight
Our proprietary clinical trial dataset captures the ripple effects of this regulatory disruption.
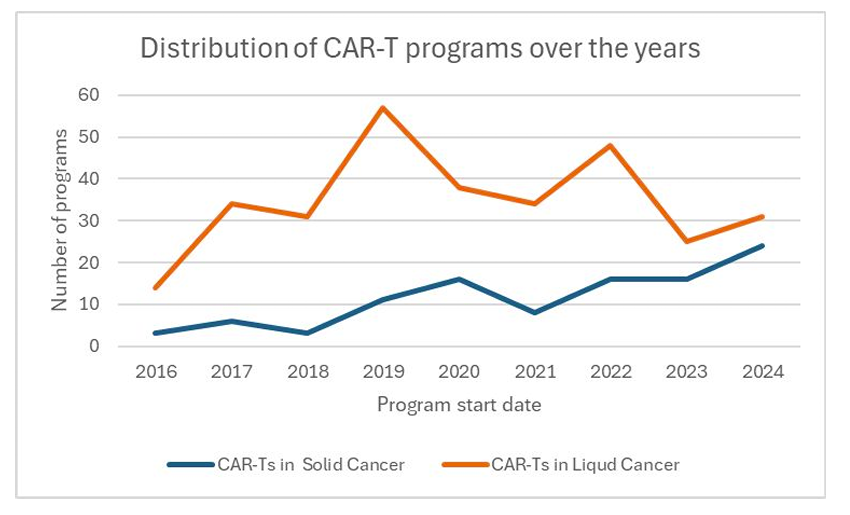
What’s now emerging—and less often discussed—is a significant and sustained rise in CAR-T programs for solid tumors. Based on our analysis of clinical program initiations from 2016 to 2024:
- While liquid tumor programs still lead in total volume, the number of new solid tumor-focused CAR-T programs has increased more than 5-fold over that period.
- In 2023 and 2024, the annual number of new solid tumor CAR-T programs is approaching parity with those in liquid cancers.
- This marks a strategic reorientation in clinical investment, not yet reflected in regulatory approvals—but clearly visible in trial pipelines.
Despite continued skepticism about feasibility in solid cancers—owing to challenges such as tumor heterogeneity, immune suppression and target accessibility—the trend data suggests that a Phase 3 trial in a solid tumor setting may be closer than anticipated. To date, no such program has reached Phase 3, but the growth curve suggests that critical mass may soon be achieved.
Seizing the Inflection Point
For portfolio leaders and clinical strategists, this trend presents a narrow window of opportunity:
- Early engagement in solid tumor CAR-T could provide a first-mover advantage in shaping regulatory expectations and clinical endpoints.
- Cross-functional planning (CMC, trial design, site selection) will be critical to overcome technical barriers in solid tumor deployment.
Acting Ahead of the Curve
Our SaaS suite of solutions supports pharmaceutical decision-makers with:
- Modality-specific development benchmarks, including transition probabilities and attrition patterns across tumor types, helping portfolio teams set realistic expectations, prioritize high-yield programs, and avoid investing in weak benchmarks.
- Predictive success modeling to estimate program viability based on trial design, indication, and sponsor profile, enabling sponsors to make smarter go/no-go decisions, reallocate resources earlier, and reduce late-stage risk.
- Signal detection for inflection points in trial volume, endpoint use, and regulatory engagement. We surface information when modalities like CAR-Ts in solid tumors shift from exploratory to investable—so you can act ahead of the curve, not behind it.
Solid tumor CAR-T isn’t just a long-term bet—it’s an active transition zone. Knowing when to move is what separates trend followers from market leaders.



